PathoSense Onboarding Journey
What to Expect During Your Implementation Journey
Duration: Your implementation journey will take about 10 weeks from start to finish. Each step builds on the last, making sure you have a smooth onboarding experience. This timeline is a guide for getting PathoSense up and running in your lab. We're here to support you every step of the way. The actual time it takes to be fully operational will depend on your team's efforts in setting up your facilities, completing training, and smoothly running all validation processes.
Your Role: Follow each step in the timeline below to explore detailed requirements, access necessary documentation, and track your progress. You'll have dedicated support throughout the entire process with regular check-ins and assistance when needed.
Support Available: Our implementation team will guide you through each phase, provide technical assistance, and ensure you meet all requirements before progressing to the next step. You'll receive personalized training and have access to our complete resource library.
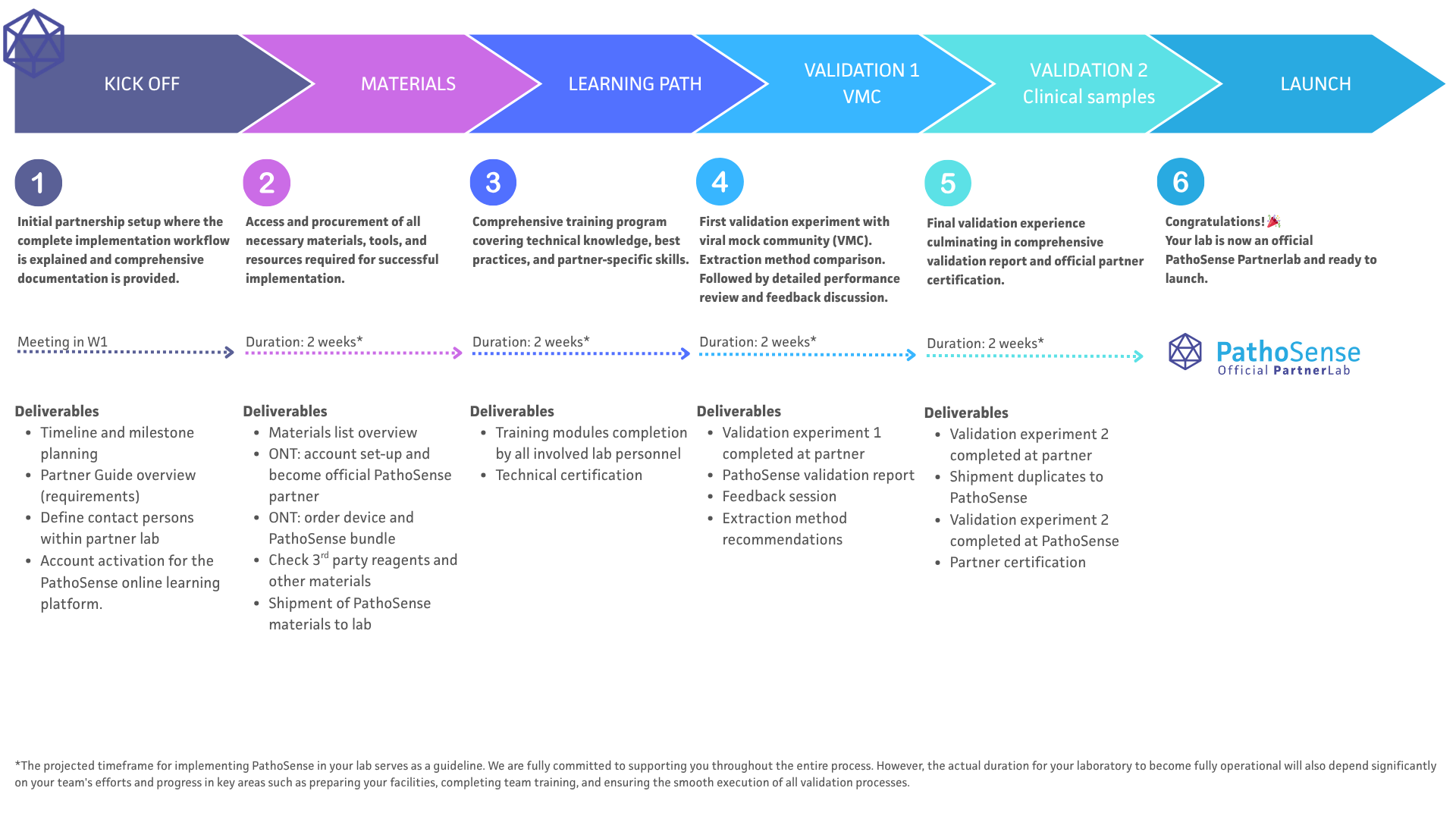
1 - Kick Off
Initial Assessment & Setup
In this first step, we organize a kick-off meeting and discuss the timeline and onboarding process, identify contact persons, and set up your account for the onboarding platform.
2 - Materials Wet lab
Equipment & Infrastructure
To fully get started, check the required laboratory equipment and order materials.
In the Complete Material List PathoSense Dx Assay ✅ , you can find all necessary equipment and materials. This includes the PathoSense materials, the ONT device and bundle, third party materials, ...
Complete Material List PathoSense Dx Assay ✅
You can find the complete list of all materials needed for the PathoSense workflow here:
PathoSense Materials 📦
Part of the required materials will be supplied directly by PathoSense. At the start of the implementation, you will receive two separate shipments:
-
One shipment on dry ice, which includes the VMC for your initial validation experiments
-
One cooled shipment with additional materials
You will receive track & trace codes by email once the shipments are on their way.
📦 More details about the shipments can be found [here].
🔁 To reorder a materials package (sufficient for 576 tests), please use [this order form].
Oxford Nanopore Technologies 🧬
Getting Started with ONT
To begin using PathoSense, your lab will need access to Oxford Nanopore Technologies (ONT) materials.
We will notify ONT that your lab is an official PathoSense partner. This allows you to purchase the dedicated PathoSense bundle through their webshop.
If your lab does not yet have an ONT account, please create one via the ONT website. Once your account is set up, ONT support will reach out to link it to PathoSense, enabling access to the required bundle.
If you don’t have a MinION device yet, you can purchase one through a local supplier or directly from ONT. We recommend choosing a starter pack that includes R10 flow cells to ensure compatibility with PathoSense workflows.
Sartorius Filter 🧪
PathoSense has a direct partnership with Sartorius for our partners.
Sartorius has been informed and will contact your lab shortly to facilitate your filter order.
SOP Aliquoting IC 🫗➗
Here you can find the SOP 'Aliquoting IC +HMB'
3 - Learning Path
Staff Training & Certification
In this step, lab staff can run through the training modules and obtain required certifications for PathoSense procedures.
To get started with the wet lab training:
4 - Validation 1: VMC
Validation of Viral Mock Community
In the first validation experiment the PathoSense workflow is conducted using a viral mock community provided by PathoSense.
This experiment serves two primary purposes:
First, it allows you to perform an intralab comparison.
Second, you have the opportunity to benchmark your laboratory's current extraction method against the extraction protocol recommended by PathoSense. This side-by-side comparison will help determine which approach yields optimal results for your specific laboratory setup.
Following the completion of this experiment, we'll schedule a results review session to discuss your findings and make any necessary adjustments to the extraction methodology moving forward.
5 - Validation 2: Clinical Samples
Clinical Sample Validation Experiment
In this step, a second validation experiment will be conducted using clinical samples collected from different animal species and sample matrices. This experiment is designed to evaluate the real-world performance of the PathoSense protocol with diverse clinical specimens.
The primary objective is to test whether the sequencing workflow performs smoothly when processing actual clinical samples, as opposed to the controlled mock community used in the first experiment.
Additionally, duplicates of benzonase-treated samples will be prepared and sent to PathoSense for parallel processing. PathoSense will run these duplicate samples, allowing for a direct comparison of results between the partner laboratory and PathoSense. This interlab comparison will help validate the consistency and reproducibility of the protocol across different laboratory environments.
6 - Launch
.png?width=2000&height=808&name=image%20(6).png)